Hematopoietic Stem Cell-Based Gene Therapy for Metachromatic Leukodystrophy (MLD)
- 領域
- Therapeutic Biologics
- Patent
- IP00825
Key Problem and Market Opportunity
- Metachromatic Leukodystrophy, commonly known as MLD, is a genetic disorder that affects the white matter, or myelin, of the brain and the central nervous system. The life expectancy is usually less than 10 years after diagnosis.
- MLD is an autosomal recessive genetic defect caused by mutations of the arylsulfatase A (ARSA) gene.
- There is no cure for MLD at present. Management of most MLD patients is usually limited to supportive care.
Key Advantages of the Technology
This invention provides an advanced hematopoietic stem cell (HSC)-based gene therapy to treat MLD, including the following:
- A modified version of lentivirus vector containing the human ARSA gene for MLD treatment
- Protocols of mass production of Lenti-ARSA for clinical application in compliance with GMP requirements
- Protocols for transduction of Lenti-ARSA into HSC
- Culturing medium and methods to maintain the multipotency of the transfected stem cells
- Clinical assessment system to determine disease severity subjected to HSC-gene therapy
Benefits
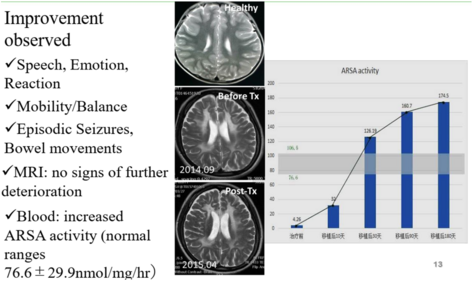
- Successful treatment for 9 patients including treatment for late-stage MLD cases which has not been achieved by other methods
- No obvious side effects
- No increased risk of tumorigenesis
Potential Product and Services
Therapeutics for treating MLD
Development Status and IP Strength
Stage of Development
- Clinically proven in 9 patients
Patents
- PCT Application No. PCT/CN2020/077088 filed on 28 Feb 2020
IP Status
- Patent application submitted