Materials And Methods For Filling Biological Cavities And Preventing Leakage Of Injected Therapeutic Agents
- 領域
- Medical Devices
- Patent
- IP00438
Key Problem and Market Opportunity
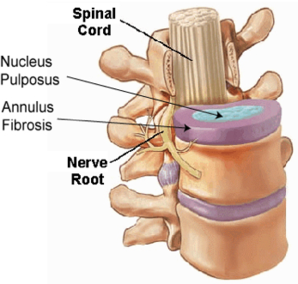
- Injection of biomolecules, genes and cells is commonly used in biological therapies. For example, intra-discal injection of biomolecules such as transforming growth factor-beta 1 has been used in the treatment of degenerative intervertebral discs (IVD). Recently, mesenchymal stem cell (MSC)-based therapy for treating degenerative discs has received much attention.
- A critical problem common to all intra-discal injection is the leakage or backflow of the injected materials through the injection portal caused by the large intra-discal pressure. The leakage of injected cells and other biomaterials negatively affects the safety and efficacy of cell-based therapy in disc degeneration. There is a need of developing improved devices for preventing leakage of injected materials in biological therapy.
Key Advantages of the Technology
- The invention introduces a collagen based plug, a specially designed set of equipment and surgical procedures for preventing cell suspensions, fluids, mixtures and gelatinous substances from leaking after injection into soft tissue such as IVD.
- The invention can reduce the leakage of therapeutic agent into a non-target site within the body of the subject. For example, the leakage of intra-discal injection of stem cells into nucleus pulposus of an intervertebral disc during cell therapy can be reduced, by delivering the filling device into the internal space or cavity created by the intra-discal injection.
Potential Product and Services
- Medical apparatus for preventing leakage of injected therapeutic agent such as drugs, genes, cell suspensions, fluids, mixtures and gelatinous substances from body of subject.
Development status and IP Strength
- Proof-of-Concept/In Vivo Study (Rabbits) Completed
- Patents have been granted in US and CN, pending in EP