Virus Like Particles (VLP) for the Diagnosis of and Protection Against Rat Hepatitis E Infection
- 領域
- Diagnostics
- Patent
- IP01019
Key Problem and Market Opportunity
- Rat hepatitis E virus (HEV-C) is extremely divergent to the usual variants of hepatitis E circulating in humans, which are often derived from swine (HEV-A). Existing hepatitis E antibody tests often miss rat hepatitis E infection and are unable to differentiate HEV-C infection from HEV-A.
- Existing commercial vaccines against hepatitis E (Hecolin) only offer limited protection against rat hepatitis E.
- Global Hepatitis E Diagnostic Tests Market is valued approximately USD 50 million in 2019.
- WHO estimated that every year there were an estimated 20 million HEV infections worldwide, leading to a 3.3 million symptomatic cases of hepatitis E, resulting in over 70,000 fatalities and 3,000 stillbirths.
Key Advantages of the Technology
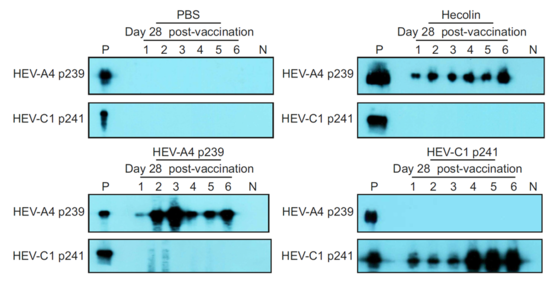
- VLP (HEV-C1 p241) based antibody test can detect and differentiate between human and rat hepatitis E infection. The VLP could be deployed in commonly used antibody assay formats such as Western Blot and ELISA.
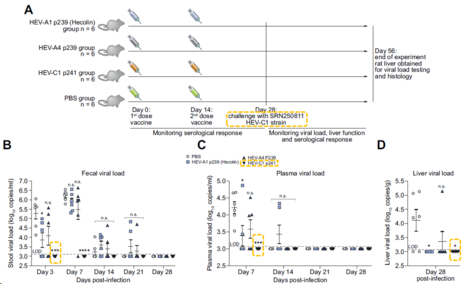
- The VLP (HEV-C 1 p241) is immunogenic in rat and administration of the VLP can protect rats against rat hepatitis E virus infection. This supports the possibility that the VLP can be used as vaccine in human.
Further Details
- Journal publication: Journal of Hepatology 2021 vol. 74 j 1315–1324 https://doi.org/10.1016/j.jhep.2020.12.028
Potential Product and Services
- Antibody tests for detection and differentiation of human and rat hepatitis E virus infection.
- Vaccine against rat hepatitis E virus.
Development Status and IP Strength
Patents
- PCT application
IP Status
- Patent application submitted