Novel Biomaterial with Ultra High Content of GAG
- 領域
- Medical Devices
- Patent
- IP00988
Key Problem and Market Opportunity
- In an aging population, intervertebral disc (IVD) degeneration is not uncommon and may cause significant reduction of quality of life due to loss of mobility
- Glycosaminoglycan (GAG): a very key component with structural, mechanical and biological functions in IVD and many other tissues.
- Advances in tissue engineering will allow patients to replace IVD with artificial discs with high GAG content.
Key Advantages of the Technology
- High GAG content biomaterial for IVD has not previously been made. This technology from HKU is the very first one with established know-how to make.
- A key component in future non-fusion spinal surgery market.
-
Benefits
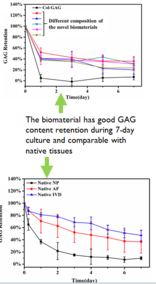
- Very high GAG to collagen ratio: >27
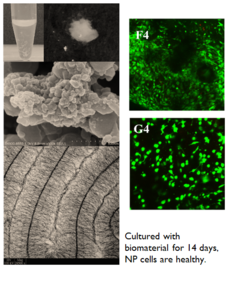
- Closely mimics native nucleus pulposus (NP) with brush-like and bead-like structures
- Primary NP cells can grow on this biomaterial very well
- Cell-embedded biomaterial has highly comparable mechanical property as native IVD
Potential Product and Services
- Fully biocompatible artificial IVD for disc replacement
- Injectable biomaterial for treatment of disc degeneration
- Base scaffold for tissue engineering which requires high GAG content matrix
Development Status and IP Strength
Stage of Development
- Production protocol has been established
- In vitro study has completed
Patents
- Filed PCT in 2021
IP Status
- Patent application submitted
Seeking
- Development partner
- Licensing