A Novel, Highly Sensitive and Specific Diagnostic Assay for COVID-19
- Field
- Diagnostics
- Reference No.
- IP00930
Key Problem and Market Opportunity
- Currently, world is facing a pandemic situation due to coronavirus outbreak. As per WHO, the positive cases of Covid-19 across the globe rose to 416,686 with 18,589 deaths in the middle of March 2020. In addition, unavailability of specific medicine or a vaccine propels the Covid-19 diagnostics market growth.
- At present, the most widely used diagnostic test for detection of Covid-19 is reverse transcriptase PCR or RT PCR. The major factors that drive the Covid-19 diagnostic market growth include significant increase in patient population across the globe and immense need of rapid diagnostics. Highly sensitive and specific laboratory diagnostics are important for controlling the rapidly evolving COVID-19 epidemic.
Key Advantages of the Technology
Technology Overview
- A novel real-time COVID-19 RT-PCR assay targeting the RdRp/Hel was developed. To avoid cross-reactivity with human SARS-CoV, the probes of the assay were designed to contain 7-9 nucleotide differences with those of human SARS-CoV. This assay is more sensitive and more specific in comparison to the RdRp-P2 assay which is used in >30 European laboratories.
- Journal publication: https://jcm.asm.org/content/early/2020/03/27/JCM.00310-20.long
Benefits
- Higher sensitivity:
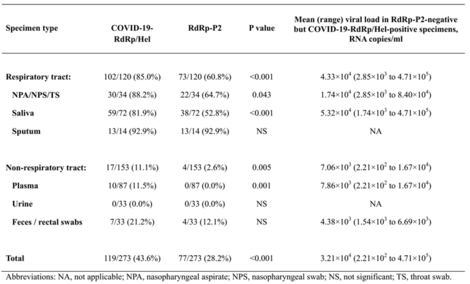
- The LOD (Limit of Detection) of the novel assay is 1-log lower than that of the RdRp-P2 assay.
- The novel assay was significantly more sensitive than the RdRp-P2 assay for the detection of SARS-CoV-2 RNA in nasopharyngeal aspirates/swabs or throat swabs (P=0.043), saliva (P<0.001), and plasma (P=0.001) specimens.
- Higher specificity: Cross-reactivity on other respiratory viruses were not found for the novel assay.
- Table 1. Comparison between the novel RdRp/Hel and the RdRp-P2 RT-PCR assays for the detection of SARS-CoV2 RNA in different types of clinical specimens from 15 patients with laboratory-confirmed COVID-19
- Table 2. Cross-reactivity compared to RdRp-P2 assay
Potential Product and Services
- Diagnostic assays for COVID-19
Development Status
Patents
- US Provisional Application No. 62/980,094 filed
IP Status
- Patent application submitted