Endosomal Acidification Inhibitors for Influenza Virus and Coronavirus Treatment
- Field
- Therapeutic Biologics
- Patent
- IP01012
Key Problem and Market Opportunity
- Influenza viruses and SARS-CoV-2 which lead to seasonal outbreaks and a pandemic respectively have consistently overwhelmed healthcare institutions. The emergence of drug resistant viruses in patients while on treatment with specific anti-influenza drugs has fueled the development of alternative therapeutic strategies. The challenge is to identify a therapy which could inhibit the viral replication while preventing the generation of drug resistant viral variants upon intervention.
- According to Global data, the therapeutic drugs market for COVID-19 is expected to increase, generating a total sales of $50.9B from 2020 to 2027. As for the global influenza market, it is estimated to reach nearly $6.5 billion by 2022, growing at a compound annual growth rate (CAGR) of 3.0% for the period of 2017 through 2022.
Key Advantages of the Technology
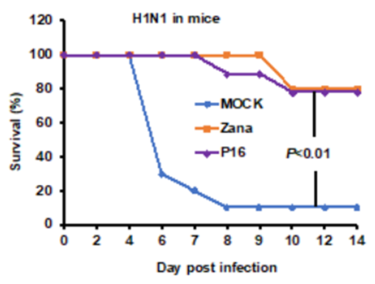
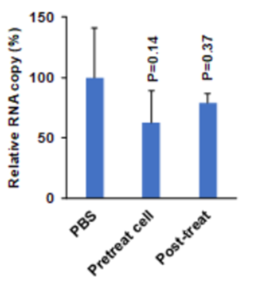
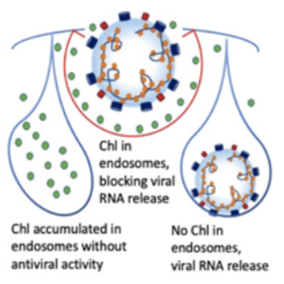
Using endosomal acidification inhibitors (Chloroquine and P16 peptide), the inventors successfully inhibit SARS-CoV-2, SARS-CoV and Influenza virus replication in vivo through intranasal administration when lung cells could be bathed in chloroquine with effective concentration.
Benefits
- Increased bio-safety (no significant hemolysis was observed in turkey red blood cells upon treatment)
- Significantly inhibit viral replication when the viruses were pretreated before viral infection
- Does not induce drug resistant gene in virus upon treatment
Potential Product and Services
Anti-viral therapy for SAR-CoV-2 and Influenza virus
Development Status and IP Strength
Stage of Development
- Chloroquine and P16 peptide have been tested in laboratory animals for its efficacy, safety and potency
Patents
- US Provisional Application No. 63/150,110
- US Provisional Application No. 63/150,141
IP Status
- Patent application submitted