A new biomarker for risk assessment and management of patients with cardiometabolic and cardiorenal syndrome
- Field
- Diagnostics
- Patent
- IP00665
Key Problem and Market Opportunity
- Cardiovascular, metabolic and renal diseases are the major culprit for high morbidity and mortality in aged population and represent the leading causes of death worldwide.
- Current biomarkers in diagnostic and prognostic of cardiac and renal abnormalities involve triglycerides (TG), HDL cholesterol, troponin, creatine kinase, natriuretic peptides, cystatin C and etc. However, sensitivity and reliability of the markers may not be always good.
- According to Frost and Sullivan, the global cardiovascular monitoring and diagnostic devices market was valued at USD 3.7 billion in 2012 and is expected to reach a value of USD 7.0 billion in 2019. The in vitro diagnostic renal biomarker market was valued at $812 million in 2014 and is expected to reach $1.1 billion by 2019.
Key Advantages of the Technology
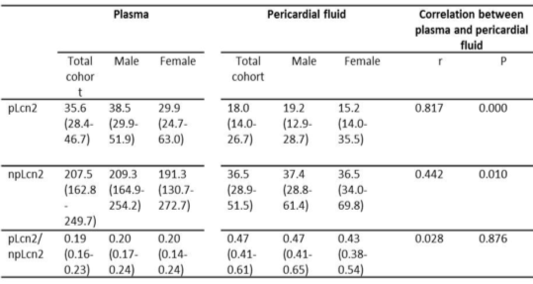
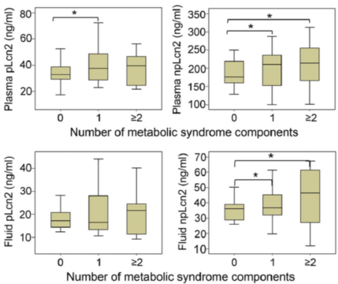
- In healthy volunteers, serum npLcn2 levels are positively correlated with heart rate, circulating triglycerides, high-sensitivity C-reactive protein and creatinine in plasma. Urine npLcn2 levels are significantly increased in subjects with metabolic syndrome.
- In cardiothoracic surgery patients, the levels of npLcn2 in circulation are higher than those in healthy volunteers and positively correlated with the levels of this protein in the pericardialfluid. The patients with heart failure show excessive expression and distribution of npLcn2 in mesothelial cells and adipocytes of the parietal pericardium, which are significantly correlated with the elevated plasma levels of npLcn2, total cholesterol, and creatinine.
- Quantitative and qualitative evaluation of npLcn2 in human biofluid samples and tissue samples can be applied for risk assessment of healthy individuals and disease management of patients with obesity-related cardiometabolic and renal complications.
Potential Product and Services
- npLcn2 as a biomarker for identification or risk assessment of cardiometabolic diseases
- Immunoassays, kits and reagents for detecting npLcn2 in tissues and biofluids
Development Status and IP Strength
- PCT/CN2016/103792 filed on 28 Oct, 2016.