An antibody for predicting Tamoxifen response in breast cancer patients
- Field
- Diagnostics
- Patent
- IP00577
Key Problem and Market Opportunity
- Tamoxifen, an antagonist of estrogen receptor (ER), is the most commonly used chemotherapeutic agent for the treatment of ER-positive breast cancer.
- However, its application and efficacy is highly limited by drug resistance. Currently, there is no available biomarker to discriminate patients who are sensitive to tamoxifen from those who are not. Therefore, almost all the patients with positive ERα status will be prescribed with tamoxifen. Some patients are resistant to this drug but by the time the clinicians realized that the drug has failed, the cancer have already spread and metastasized.
- Breast cancer is the most common cancer in women worldwide. The number of new cases of female breast cancer is about 124.9 per 100,000 women per year. About 80% of all breast cancers are ER positive while 30% of these cancers are resistant to tamoxifen therapy, either before or during the treatment.
- AcuteMarket reports indicated that the tamoxifen market was valued at USD 675.7 Million in 2015, and is expected to reach USD 683.6 Million by 2022, expanding at a CAGR of 0.07% from 2016 to 2022.
Key Advantages of the Technology
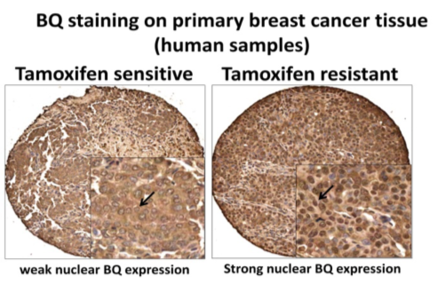
- The present invention identified a splice variant of NCOR2/SMRT, BQ323636.1, having association with tamoxifen resistance. A novel monoclonal antibody specific for this splice variant as a predictive marker for tamoxifen resistance is created.
- Overexpression of BQ323636.1 conferred resistance to tamoxifen both in vitro using cell lines and in vivo using nude mice model.
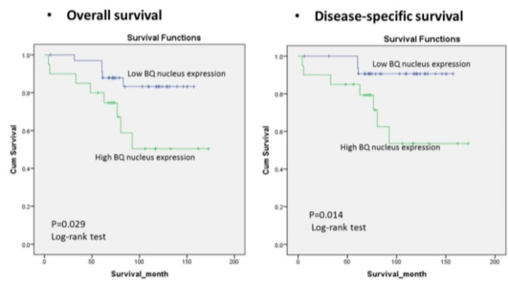
- IHC staining using this antibody on tissue microarray (TMA) constructed from 100 cases of archived breast cancer patients’ paraffin blocks successfully showed that BQ323636.1 overexpression could predict tamoxifen resistance (p=0.007) (tamoxifen resistance being defines as patients who received tamoxifen and later developed relapse or metastasis) and was associated with poor survival (p= 0.029 for overall survival, p=0.014 for disease specific survival).
- Advanced prediction of Tamoxifen responses using of BQ expression as a biomarker can facilitate correct choice of treatment and may improve disease outcome.
Potential Product and Services
- anti-BQ323636.1 monoclonal antibody as a diagnostic tool
Development status and IP Strength
- PCT application No. PCT/CN2016/097131 filed on 30 Aug 2016.