Delivery and expression systems for anti-viral therapeutic molecules
- Field
- Therapeutic Biologics
- Patent
- IP00775
Background and Market Opportunity
- Seasonal influenza virus annually causes over 3–5 million cases of severe illness with about 0.25 million deaths globally.
- Limited efficacy of current antivirals and antiviral-resistant mutations impairs anti-influenza treatment.
- Use of Defective interfering genes (DIG) is a promising strategy to treat influenza as it neither generates new reassortants nor neutralizes antibodies.
- However, the use of DIGs is dependent on safe and efficient delivery to the cells at the site of infection.
- One of the promising delivery vector systems for DIGs are peptides. Peptides have established their role as delivery vectors in humans due to their low toxicity and absence of toxic metabolites.
- According to the Acute Markets report, the global influenza therapeutics market will swell to $1.2 billion by 2025, from $600 million in 2016.
Technology Overview and Key Advantages
- A dual-functional system with both gene delivery and antiviral ability in vivo.
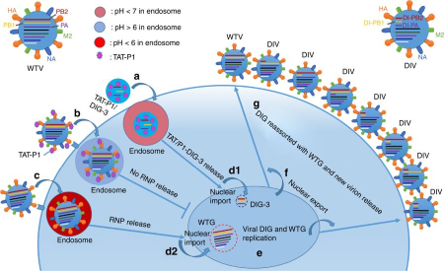
- This system includes DIG-3, which outcompetes full-length viral genes and generates DIV, along with a dual-functional peptide vector consists of two components, HIV-1 Tat (TAT) and P1 peptide.
- This dual function peptide vector, TAT-P1, efficiently delivers DIG-3 to inhibit viral replication and also directly inhibits viral replication by preventing endosomal acidification.
- TAT-P1/DIG-3 interferes with the replication of diverse subtypes of influenza virus at two steps within one life cycle of virus, as shown in Fig. 1.
Advantages
- Broad antiviral activity with a low likelihood of inducing antiviral resistance.
- Protect from lethal virus challenge
- TAT-P1/DIG-3 conferred significantly better mouse survival than that of zanamivir.
Potential Product and Applications
- Transfection vectors with wide applications in gene antiviral strategies for treating viral respiratory diseases.
- Treatment of avian and seasonal influenza virus infections.
Development Status and IP Strength
- US Provisional Application No. 62/668,872 filed on 09 May, 2018