Metallodrug Ranitidine Bismuth Citrate as an Anti-COVID 19 Agent
- Field
- Therapeutic Chemicals
- Reference No.
- IP00960
Key Problem and Market Opportunity
- Background: Currently, therapeutic options for COVID-19 are limited. Remdesivir, a broad-spectrum antiviral drug, has been approved by FDA for SARS-CoV-2. However, global shortage of the drug, its relatively high price and lack of significant clinical benefits in severe cases, are factors that have limited its wider applications. Therefore, greater efforts are needed to explore more drug candidates, which hopefully could open the way to alternative treatment strategies against the disease through some readily available channels.
- Technology Overview: Drug candidate (RBC) targets the vital non-structural protein 13 (Nsp13), a viral helicase essential for SARS-CoV-2 to replicate, by displacing the crucial zinc(II) ions in the zinc-binding with Bismuth-ions, to potently suppress the activity of the helicase.
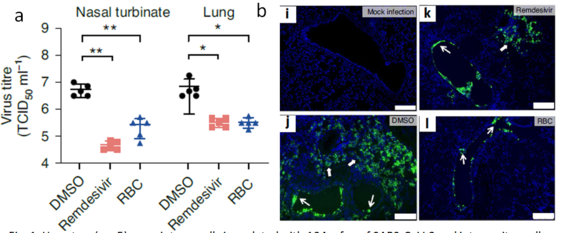
- Fig.1: Hamsters (n = 5) were intranasally inoculated with 104 p.f.u. of SARS-CoV-2 and intraperitoneally given either DMSO (vehicle control), RBC (150 mg/kg) or remdesivir (15 mg/kg) for four consecutive days. (a) Virus titres; (b) Viral N protein expression in lung tissue sections 1
Further Details
Shuofeng Yuan1,6et al., Nature Microbiology volume 5, pages1439–1448(2020)
Key Advantages of the Technology
- Suppresses SARS-CoV-2 replications to reduce viral loads by ~100 folds in both the upper and lower respiratory tracts
- Has a high therapeutics index (RBC at 975 vs. Remdesivir at 129)
Potential Product and Services
- Antiviral drugs, especially SARS-CoV-2
Development Status
Patents
- US provisional
IP Status
- Provisional patent